
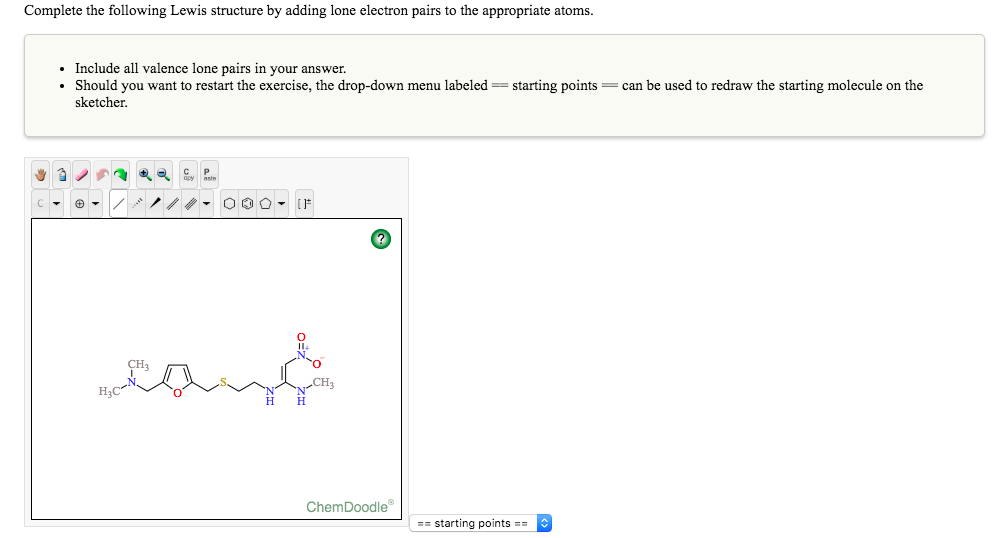
The bonds and the spare electrons will be indicated (or can be easily found from) the molecule’s Lewis structure. This way, carbon has 4, oxygen has 6, and hydrogen has 1 valence electrons. So if were to add up all these electrons here we have eight from carbon atoms. So the acetate eye on is usually written as ch three c o minus. But we know that hydrogen can form only a single bond, this means H cannot be the central atom. Formal charge Valence Electrons Sticks Dots The number of valence electrons equals to the element’s group (column) in the periodic table. Now were going to work on Problem 41 from chapter five in this problem, whereas to draw Louis structure for the acid ate ion, including all resident structures, and to indicate which Adams will have a charge. If you sum all these valence electrons, you will get 10. If you look on the periodic table, you will notice that H has one valence electron, C has 4, and N has 5. To draw the Lewis structure for HCN, we will first calculate the total number of valence electrons. Now, let’s apply the above rules to predict the best Lewis structure for the molecule, ChemDoodle Web Components allow the wielder to present publication quality 2D and 3D graphics and animations for chemical structures, reactions and spectra.
#CHEMDOODLE HELP FORMAL CHARGE HOW TO#
How to decide the correct Lewis structure after assigning formal chargesĪfter assigning formal charges, we again apply the following rules to identify the correct Lewis structure: If it is a molecular ion, then the sum of all the formal charges must equal the ionic charge. If it is a neutral molecule, then the sum of all the formal charges must equal zero.

ChemDoodle Web Components allow the wielder to present publication quality. Once we add all the formal charges for the atoms in the Lewis structure, we should get a value equal to the actual charge of the molecule or ion. The ClO4-Lewis structure is a good structure to help you understand Nov 25. Assign half of the bonding electrons to each atom in the bondĪfter applying the rules outlined above to each atom in the Lewis structure, we will then use the following formula to calculate the formal charge of each atom: How to calculate formal charge chemdoodle 2d v11.Assign all lone pairs of electrons to the atom on which we find them.To determine the formal charge for an atom, we usually follow these rules: When that happens, we usually assign formal charges to the bonded atoms to help determine the correct Lewis structure. Sometimes we can write more than one Lewis structure for a particular ion or molecule. Types of electric charges - Basic Physics Tutorialsįormal charge is the charge we assign to a bonded atom if the bonding electrons were shared equally between the bonded atoms.


 0 kommentar(er)
0 kommentar(er)
